Part 5: Mechanical Intravenous Infusion Pumps
- EMR Discovery
- May 14, 2020
- 2 min read
This post is Part 5 of a series based on Nursine Jackson’s article, “What about that Device Data?”.
Mechanical Intravenous Infusion Pumps
Mechanical infusion pumps are another source of information that may not present in the medical record except for an audit trail of device data.
An infusion pump is a medical device that delivers fluids, such as nutrients and medications, into a patient’s body in controlled amounts. Infusion pumps are in widespread use in clinical settings such as hospitals, nursing homes, and in the home. Many infusion pumps are equipped with safety features, such as alarms or other operator alerts that are intended to activate in the event of a problem.
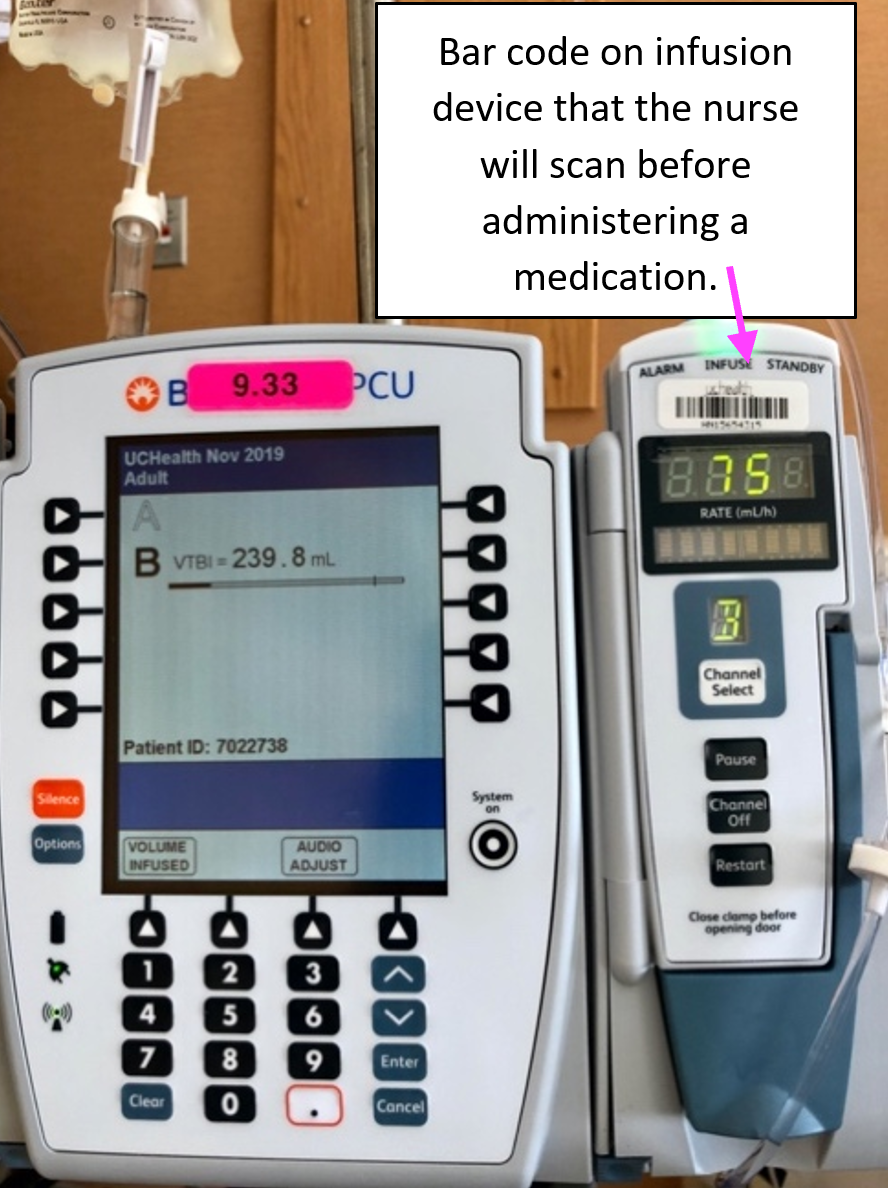
For example, some pumps are designed to alert users when air or another blockage is detected in the tubing that delivers fluid to the patient. Some newer infusion pumps, often called smart pumps, are designed to alert the user when there is a risk of an adverse drug interaction, or when the user sets the pump’s parameters outside of specified safety limits.[1] There can also be a bar code on the infusion device that the nurse scans before administering the medication.
The settings, alarms, identities of caregivers who responded to alarms and alerts, and workstation from which they responded, may be preserved and available on the device audit trails. In the image above, the larger device was infusing the main IV fluids and the smaller device to the right was used to infuse supplemental electrolytes, antibiotics, and other medications administered intermittently.
In the labor and delivery setting, sophisticated infusion pumps used to infuse Pitocin utilize software that preserves and stores device data remotely, often in Cloud storage – not on the device. Again, depending on the focus of your case, this device data yields potentially valuable eDiscovery that may tell a story very different from the entries. This oftentimes involves late entries made by the nurse into the labor and delivery flowsheet after the bad outcome is known.
Stay tuned for Part 6 of this series, “Pitocin Pump Case Study”.
[1] U.S. Food & Drug Administration/General Hospital Devices and Supplies/Infusion Pumps. Retrieved from https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/infusion-pumps

_transp_775x681.png)



Comments